Introduction
Elevated CO2 levels are common among patients in the pediatric sleep lab. In fact, research shows that up to 46% of children with sleep-disordered breathing can have a nocturnal change in CO2 of more than 10 mmHg.¹ In the Pediatric Sleep Center at Nemours Children’s Hospital, transcutaneous CO2 monitoring is a valuable tool that we use for both routine and complex cases. By providing continuous monitoring of CO2, this tool enables our team to see patients’ ventilatory status throughout the night and monitor for hypoventilation.
The following four case studies from our unit showcase how we leverage this technology to guide titrations, support clinical decision-making, and provide effective follow-up care for many patients seen in our lab.
When We Prefer Transcutaneous Monitoring
In many clinical scenarios in our sleep lab, transcutaneous monitoring is preferred over capnography for CO2 measurement. These situations include:
Respiratory Support
Mouth-Breathers
Nasal Congestion or Secretions
Breastfeeding Infants
Facial Deformities
Sensory Sensitivities
Stomach Sleepers or Restless Sleepers
Inpatient Studies
Case Studies
Case Study 1
Patient Profile: Age 5, sleep apnea, snoring, severe OSA (AHI=47), low SpO2 (51%)
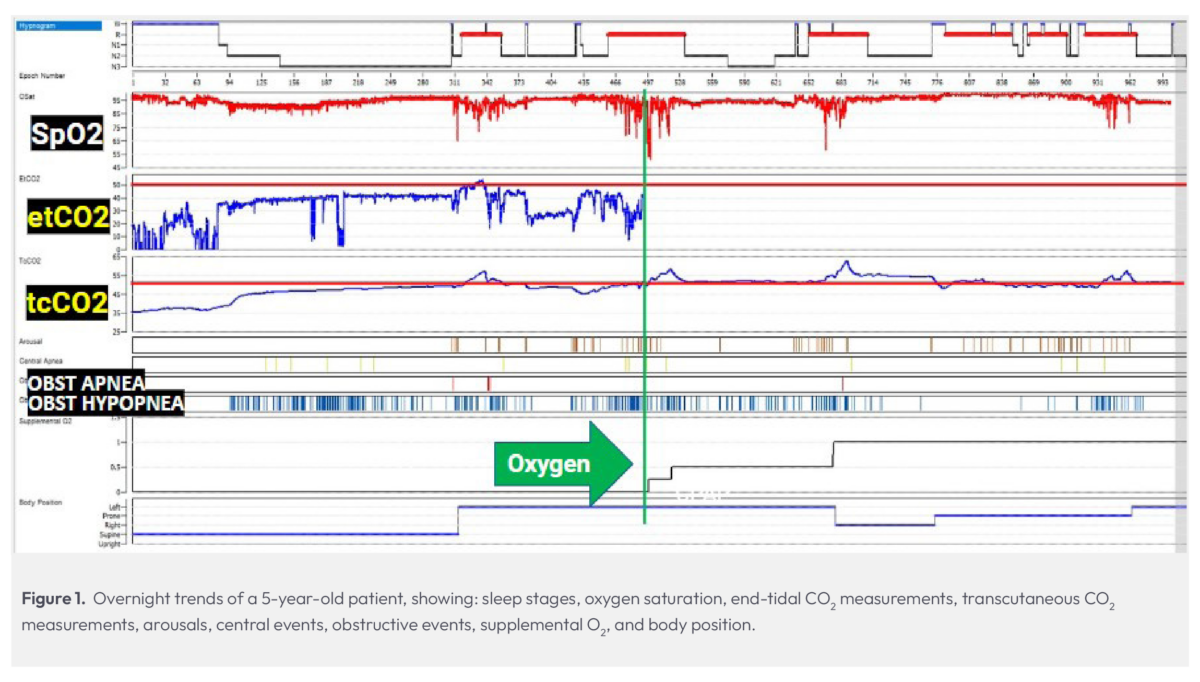
In this split-night study, a 5-year-old experiencing sleep apnea and snoring presented a challenging scenario. Despite the concerning results of a previous study, the child’s parents were unconvinced of the necessity for CPAP therapy.
During our assessment, this child exhibited an apnea-hypopnea index (AHI) of 47, indicating frequent struggles to breathe throughout the night, and a low oxygen saturation level of 51%. Upon seeing this child’s desaturations while in REM, oxygen supplementation was initiated, foregoing CPAP therapy due to the parents’ refusal.
Given the patient’s age and size, we utilized an infant cannula for oxygen delivery. Upon administering oxygen (indicated by the green line in Fig. 1), we had a complete loss of end-tidal CO2 monitoring capability.
A common concern when initiating oxygen therapy is that it may lead to an elevation in CO2 levels, which highlights the importance of maintaining visibility to CO2 — this is an established policy in our lab. Fortunately, with the transcutaneous monitor in place, we were able to continue watching CO2 levels despite the loss of etCO2, noticing that the patient spent much of the night with elevated CO2. Ultimately, the evidence from transcutaneous CO2 monitoring, alongside other indicators of hypoventilation, desaturations, and obstructive events, helped us demonstrate to the parents the necessity of CPAP therapy for their child and successfully gain their compliance with a treatment plan.
Case Study 2
Patient Profile: Age 6, adenotonsillar hypertrophy, snoring, allergic rhinitis, severe OSA
The 6-year-old patient in this split-night study had a history of severe OSA, enlarged tonsils and adenoids, snoring, and rhinitis. Usually, the preferred treatment for such patients includes the removal of their tonsils and adenoids. However, surgery may be postponed due to scheduling conflicts or some parents might opt against it altogether. In these cases, CPAP therapy is often recommended either as an interim solution while waiting for surgery or as a longer-term alternative if surgery is declined.
In this particular case, we conducted a split-night study (Fig. 2). After fitting the CPAP mask over the end-tidal cannula, we noticed some washout of CO2 readings. We had seen some variation in the end-tidal readings before splitting to CPAP, but with the mask on, etCO2 dropped significantly.
Throughout the night, our transcutaneous monitor provided more stable readings. As REM sleep increased, we observed a gradual increase in CO2, which was in contrast to what the concurrent end-tidal readings were indicating.
During the last REM period, a cluster of obstructive events caused the patient’s tcPCO2 reading to rise significantly. This prompted the technologist to adjust settings to mitigate the events and manage the patient’s increasing CO2 and decreasing SpO2. Both parameters improved following the adjustments, indicating a successful titration. Although the spike in obstructive events would have prompted titration of this patient’s pressures, transcutaneous monitoring gave us confirmation of increasing CO2 during severe events and showed a return to baseline after pressures were optimized.

Case Study 3
Patient Profile: Age 19, post-adenotonsillectomy, premature ventricular contractions (PVCs), Trisomy 21, cerebral palsy, severe OSA with BiPAP and oxygen dependency
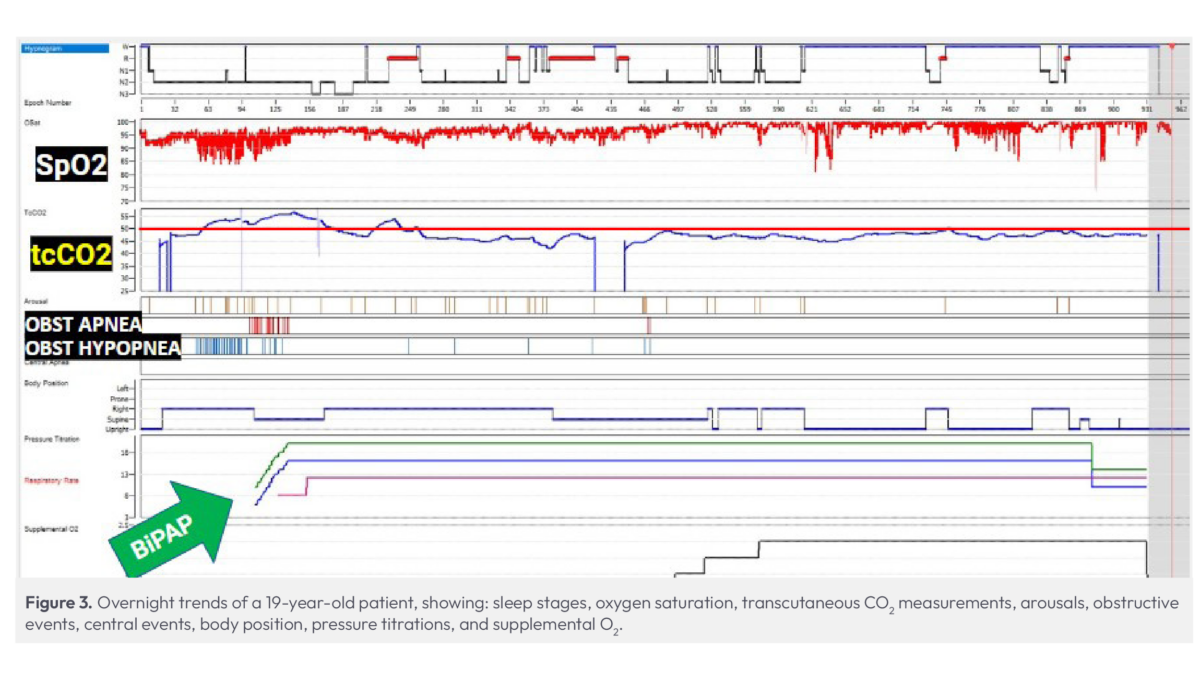
This 19-year-old patient with Down syndrome had previously undergone a tonsillectomy and adenoidectomy and presented with a history of OSA and premature ventricular contractions (PVCs). Based on previous studies, we expected a routine BiPAP titration.
For this study we employed transcutaneous monitoring, bypassing end-tidal monitoring due to the use of BiPAP (Fig. 3). Immediately, the patient’s tcPCO2 readings rose above 50 mmHg, accompanied by a significant number of events.
Despite initiating treatment at their usual BiPAP settings, the patient’s CO2 levels remained high, necessitating further titrations to open the airway. With continuous visibility to CO2 levels to guide us, we were able to achieve our target CO2 level of under 50 mmHg and determine the optimal CPAP settings for this patient.
Case Study 4
Patient Profile: Age 15, OSA, spinal fusion, chromosomal abnormality, chronic respiratory failure
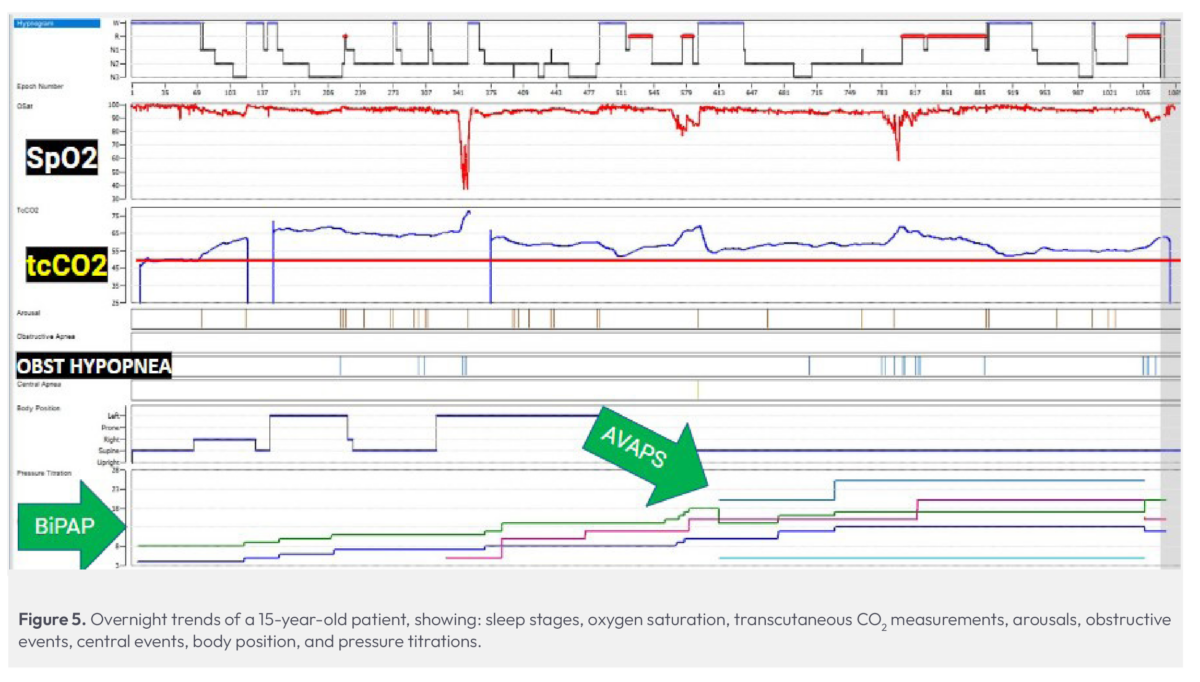
This 15-year-old patient with chromosome abnormalities presented with chronic respiratory failure and a history of OSA. Initially, their IPAP and EPAP were set to 8 cmH2O and 4 cmH2O, respectively, and we anticipated a standard BiPAP titration. Despite the absence of observed events or obstructions, CO2 levels quickly escalated into the 50 – 60 mmHg range once the patient fell asleep (Fig. 5). This unexpected rise prompted the technologist to check the site and recalibrate the sensor; however, the CO2 levels continued to climb, reaching the mid-70s (mmHg).
Given the unusual spike in CO2 levels, despite only a few events, the technologist switched to a different transcutaneous monitor to further verify the readings were correct. Although the CO2 levels decreased slightly during wake periods, they remained elevated throughout the night, consistently exceeding 50 mmHg. Following our protocol, the patient was titrated, eventually ending up on AVAPS-ST in an attempt to lower their CO2. Despite adjustments throughout the study, we were unable to reduce CO2 below 50 mmHg; although prior history was unavailable for this patient, we suspected that they were a chronic CO2 retainer, which was verified during follow-up care.
By morning, CO2 levels reached their lowest point at 56 mmHg and optimal tidal volumes were achieved on AVAPS-ST therapy. This showed surprising progress compared to overnight monitoring, where CO2 levels averaged between 60 and 70 mmHg and reached a peak of 77 mmHg for the night.
Our team was surprised at the severity of this patient’s hypercapnia, particularly given how few obstructive events they experienced throughout the night. Instead, this patient’s challenges had more to do with their respiratory failure. Without the transcutaneous CO2 monitor, we would have been blind to the severity of their condition.
More from Lisa
References
1. Pautrat, J., et al. Carbon Dioxide Levels During Polygraphy in Children with Sleep-Disordered Breathing. Sleep Breath. 2015.