Introduction
In neonatal intensive care units (NICUs), blood sampling, including arterial blood gas (ABG) tests and capillary blood tests (commonly known as a ‘heel stick’), plays an important role in patient monitoring. These tests help care teams monitor a variety of parameters, including blood pH levels and concentrations of vital gases like carbon dioxide (CO2) and oxygen (O2).
In the NICU, monitoring CO2 is particularly crucial, especially for premature infants. These values serve as indicators of both cerebral blood flow (CBF) and ventilation status. Safeguarding the brain from conditions related to uncontrolled CBF, like intraventricular hemorrhage (IVH), and the lungs from issues related to inadequate ventilation support, are two main initiatives in the NICU. Given this, vigilant monitoring of CO2 becomes essential for keeping both of these priorities in balance.
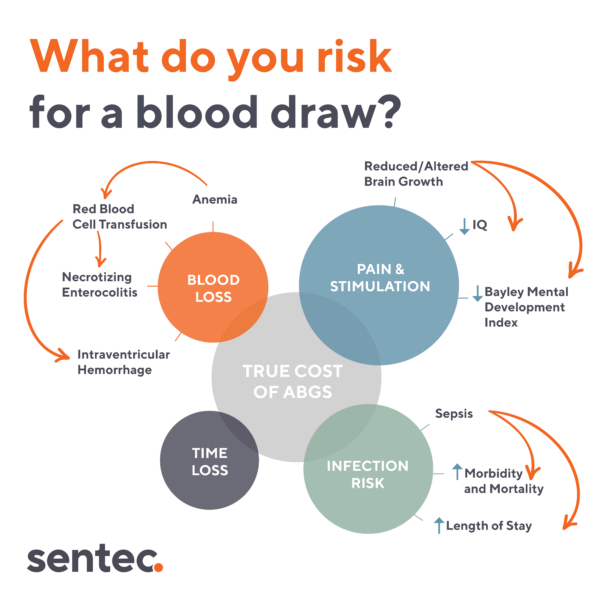
While blood samples are considered routine in neonatal care, with ABGs even considered the “gold standard” given their accuracy, they are not without drawbacks and limitations. Neonatal blood sampling, especially when performed repeatedly, carries a significant cost, not just monetary, but also in terms of short and long-term patient outcomes. Blood draws have been associated with blood loss, infection, and pain in neonatal patients. Furthermore, the time elapsed between sample collections often adds another layer of complexity, as valuable intervention time can be lost.
Here, we will explore the layered costs associated with neonatal blood sampling, shedding light on the often-underestimated challenges that arise.
Blood Loss
In the NICU, blood loss is a significant concern when it comes to blood sampling. An analysis revealed that very low birth weight (VLBW) infants undergo an average of nearly 57 blood gas measurements over the course of a week.1 This is particularly concerning for neonates, whose limited blood reserves make them exceptionally vulnerable to even small amounts of blood loss.
According to one study, this weekly laboratory testing can result in a loss of approximately 15-30% of a neonate’s circulating blood volume, equating to 11 to 22 mL per kilogram of weight. To put this in perspective, “extracting 6-7 mL of blood from a 1 kg infant is equivalent to a 450 mL blood loss in an adult.”2
For neonates, this amount of blood loss can significantly impact their overall health. Phlebotomy stands out as a primary nonphysiologic catalyst for anemia of prematurity, as evidenced by a direct relationship between the volume of blood drawn and the subsequent blood transfused.3,4 It’s imperative to acknowledge that in these delicate patients, the extracted blood must also be replaced. However, the act of transfusion in neonates poses a set of its own risks, ranging from infection, vascular overload, lung injury, and sensitization.5
Pain & Stimulation
Obtaining a neonatal blood sample, whether through a capillary heelstick, arterial puncture, or other type of blood draw, is a painful and distressing experience for neonates. Beyond the immediate discomfort, these procedures and other sources of pain in the NICU have consistently demonstrated adverse effects on long-term neurological and developmental outcomes.
Research has unveiled a troubling connection: infants subjected to a high number of neonatal skin breaks, often linked to blood draws and other invasive procedures, display lower mental development indexes when evaluated at 8 and 18 months of age.6 This detrimental influence continues to cast its shadow into the long term, affecting children even into their school years. Studies have shown negative effects on visual-perceptual skills at 7 years of age, as well as cognitive and behavioral outcomes at 8 years.7,8
The implications of these findings underscore the need for strategies to mitigate pain and discomfort experienced in the NICU.
Infection Risk
Obtaining a neonatal blood sample involves puncturing an artery or the skin of the heel, which inherently introduces the risk of infection. This risk is especially worrisome in the NICU, where infection prevention is a top priority as a result of the unique vulnerability of this patient population. NICUs worldwide have reported a hospital-acquired infection rate of approximately 10.7%, underscoring the prevalence of this concern among neonates.9
For certain infants, the necessity for frequent blood gas monitoring may require the placement of a central line. While this streamlines the testing process and reduces the number of punctures needed, it simultaneously elevates the already-high risk of infection, notably the potential for a central line-associated bloodstream infection (CLABSI). CLABSIs are not only associated with longer hospital stays, but are also a frequent culprit for sepsis in neonates, a leading cause of morbidity and mortality within this population.9,10
Oftentimes, infection control protocols and initiatives targeting CLABSIs impose limitations on both the frequency of draws that can be taken from a line and the duration in which they can be placed. While these initiatives do target reducing the risk of infection, they can consequently reduce the visibility of crucial parameters such as CO2 levels, adding yet another layer to the challenge.
Time Loss
A major drawback of blood sampling lies in its inability to continuously measure vital parameters. Instead, it offers only snapshots of a patient’s respiratory and metabolic status at individual moments in time, minimizing the ability to predict what’s to come. This limitation becomes crucial in the unpredictable NICU environment, where a patient’s condition can change rapidly, necessitating vigilant monitoring.
Relying solely on blood sampling for visibility can lead clinicians to miss fluctuations or trends in critical parameters that occur between samples. This visibility gap can result in delays in initiating necessary interventions or adjustments in treatment plans.
This information gap is particularly critical when monitoring CO2 levels in neonates, as this parameter is integral to assessing lung and brain health. Fluctuating levels of CO2, if not promptly detected and managed, can lead to IVH – bleeding in the brain’s ventricles. Hence, the inability of blood sampling to offer real-time, continuous monitoring of CO2 levels poses significant constraints in the proactive management of neonates in the NICU.
Division of Neonatology, Erasmus MC – Sophia Children’s Hospital, University Medical Center Rotterdam
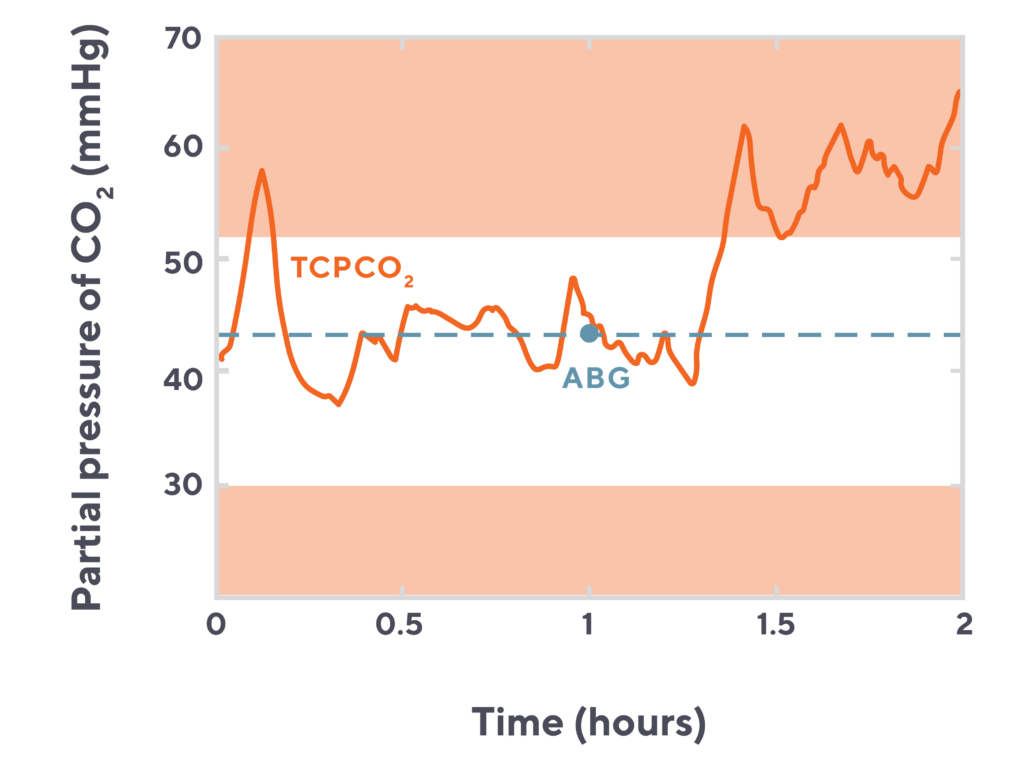
Visualizing the time lost with blood sampling
This chart of a neonatal patient’s CO2 over a two hour period highlights just how much and how quickly CO2 levels can fluctuate. How many much time spent at an elevated CO2 level might be missed by relying solely on the blood draw (orange dot) without transcutaneous monitoring (blue line).
Addressing the Downsides of Neonatal Blood Sampling
While blood sampling is crucial in the NICU, its drawbacks require careful consideration. NICUs can take proactive steps to minimize the downsides of frequent blood draws and further optimize care:
- Develop guidelines for reducing painful procedures: Establishing clear, evidence-based guidelines can help to clarify when blood draws are necessary and when noninvasive monitoring methods can be employed instead.11
- Implement infection control measures: Implementing stringent infection control measures, including rigorous hand hygiene, aseptic techniques, and proper site care can minimize the risk of infection in the NICU.12
- Use adequate analgesia: Ensuring that neonates receive appropriate analgesia or pain relief measures before, during, and after the procedure, can help minimize the pain associated with ABGs. This may involve using topical anesthetics, gentle comforting techniques, and practicing kangaroo care.114
- Bundle care interventions: Timing routine medical interventions with other care procedures can help reduce disruptions at the bedside, thereby reducing the cumulative discomfort for neonates.11
Embracing Noninvasive Approaches in the NICU
The adoption of noninvasive approaches in the NICU can be an impactful step towards mitigating the challenges associated with repeated blood testing. For example, transcutaneous CO2 monitoring offers an effective method for measuring CO2 values noninvasively through the skin. By supplementing blood draws with a method like transcutaneous monitoring, healthcare providers can reduce the need for invasive procedures, diminishing the associated risks of infection and minimizing patient discomfort.
Moreover, the impact of transcutaneous monitoring extends beyond infection prevention and pain reduction. This continuous monitoring method can play a crucial role in safeguarding neonatal health by closely monitoring CO2 levels in real-time. This allows for proactive management of this parameter, enabling the early detection of fluctuations and trends that might otherwise go unnoticed with periodic blood gas analysis.
While blood sampling undeniably holds significance in neonatal care practices, the incorporation of transcutaneous monitoring and other noninvasive techniques can support NICUs in providing gentler, less invasive, and more proactive care.
References:
- Alves-Dunkerson, J.A., et al. Cost analysis of a neonatal point-of-care monitor. Am J Clin Pathol. 2002.
- Carroll, P.D., Widness, J.A. Nonpharmacological, blood conservation techniques for preventing neonatal anemia–effective and promising strategies for reducing transfusion. Semin Perinatol. 2012.
- Widness, J.A. Pathophysiology of Anemia During the Neonatal Period, Including Anemia of Prematurity. Neoreviews. 2008.
- Valieva, O.A., et al. Effects of transfusions in extremely low birth weight infants: a retrospective study. J Pediatr. 2009.
- Whitehead, N.S., et al. Interventions to prevent iatrogenic anemia: a Laboratory Medicine Best Practices systematic review. Crit Care. 2019.
- Grunau, R.E., et al. Neonatal Pain, Parenting Stress and Interaction, In Relation To Cognitive And Motor Development At 8 And 18 Months In Preterm Infants. Pain. 2009.
- Doesburg, S.M., et al. Neonatal Pain-Related Stress, Functional Cortical Activity and Visual-Perceptual Abilities In School-Age Children Born At Extremely Low Gestational Stage. Pain. 2013.
- Chau, S.M.Y, et al. Hippocampus, Amygdala, and Thalamus Volumes in Very Preterm Children at 8 Years: Neonatal Pain and Genetic Variation. Front Behav Neorosci. 2019.
- Zingg, W., et al. Health-care-associated infections in neonates, children, and adolescents: an analysis of paediatric data from the European Centre for Disease Prevention and Control point-prevalence survey. Lancet Infect Dis. 2017.
- Karagiannidou, S., et al. Attributable length of stay and cost for pediatric and neonatal central line-associated bloodstream infections in Greece. J Infect Public Health. 2019.
- Hall, R.W., et al. Pain management in newborns. Clin Perinatol. 2014.
- Johnson, J., et al. Infection Prevention in the Neonatal Intensive Care Unit. Clin Perinatol. 2021.