Pain in the NICU
Patients in the NICU are among the most delicate residents in the hospital, yet studies have shown as many as 17 painful procedures being performed on neonates each day.1 Many painful procedures are related to blood draws for lab tests used to monitor patients and titrate support. However, more frequent blood draws lead to increased instances of pain, which can result in both short- and long-term adverse outcomes for these fragile patients. It is essential to the quality of life and long-term outlook of these preterm infants that a comprehensive approach is taken to address pain in the NICU setting.
SCROLL
Neonates in the NICU may experience as many as 17 painful procedures per day.1
Neonates in the NICU may experience as many as 17 painful procedures per day.1
Average number of painful procedures performed in the NICU per day.
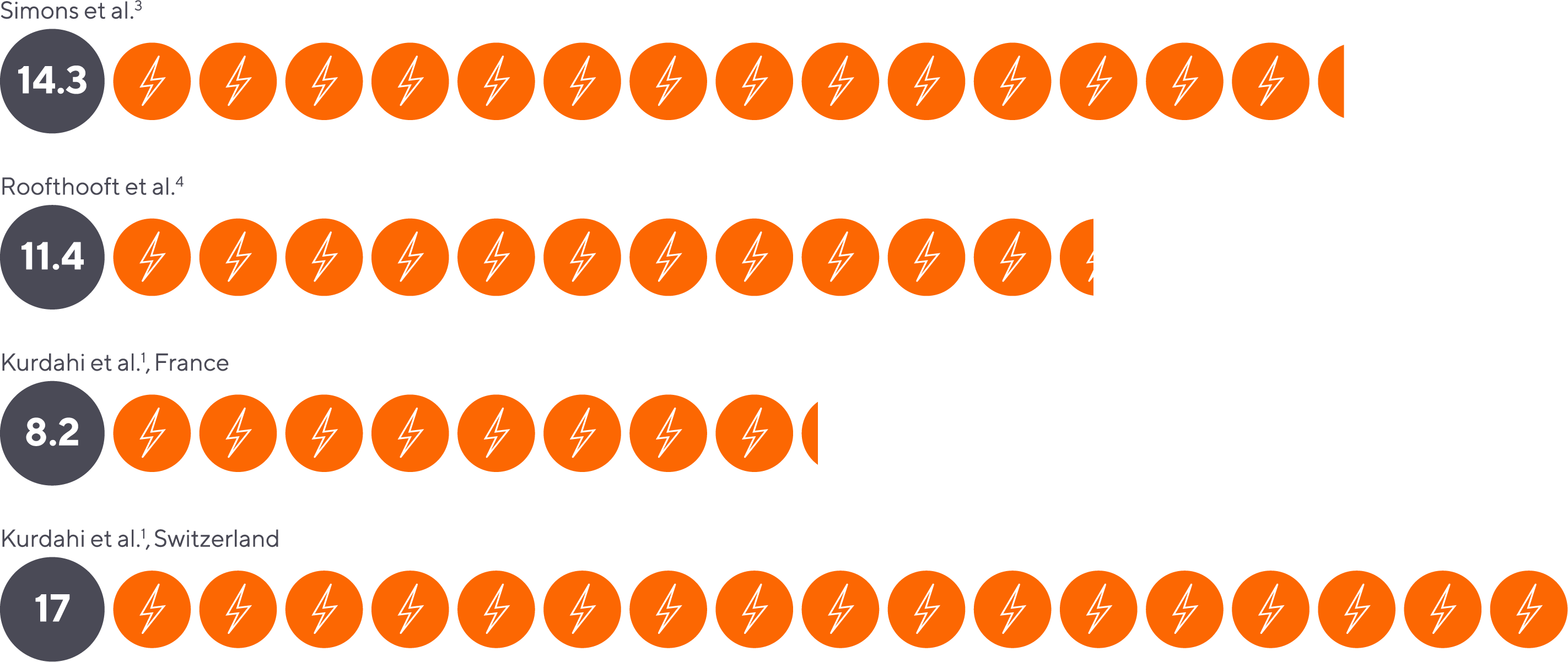
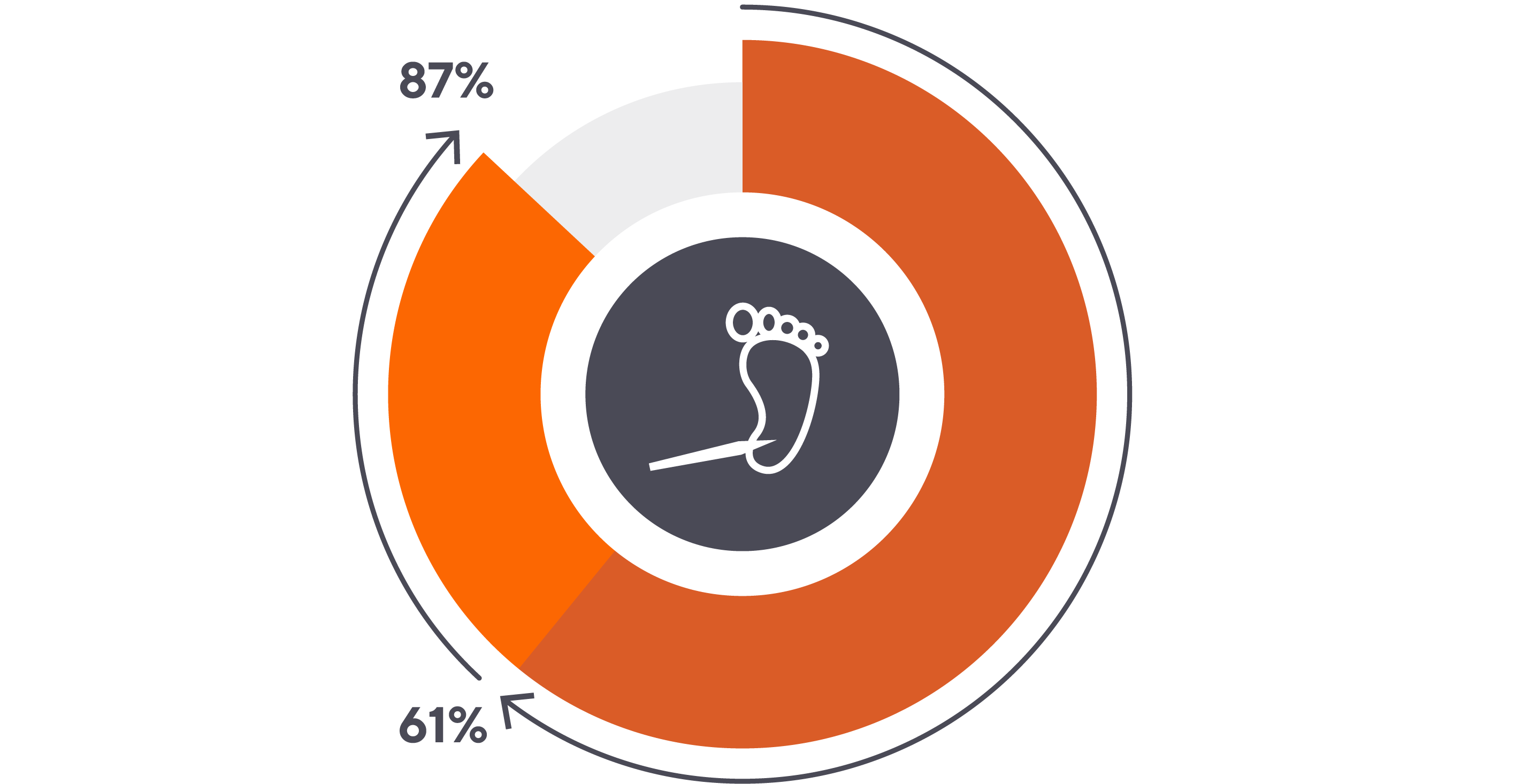
Of these painful procedures, heel sticks are the most common, comprising 61% to 87% of the invasive procedures performed on ill infants.2
Of these painful procedures, heel sticks are the most common, comprising 61% to 87% of the invasive procedures performed on ill infants.2
Preterm infants are more sensitive to pain, in part because they lack the neuro-development to comfort themselves.5

Preterm infants are more sensitive to pain, in part because they lack the neuro-development to comfort themselves.5
Analgesics are rarely given for blood sampling, and few seem to be effective.2,6,7,8
Analgesics are rarely given for blood sampling, and few seem to be effective.2,6,7,8
In addition to experiencing pain in the NICU, painful procedures in neonatal infancy may have short and long-term consequences, from increased blood pressure1 to adverse neurological outcomes.9
In addition to experiencing pain in the NICU, painful procedures in neonatal infancy may have short and long-term consequences, from increased blood pressure1 to adverse neurological outcomes.9
The short-term consequences of pain are well documented. An increase in HR, a decrease in SpO2, heart rate variability, blood pressure fluctuations and increased secretion of stress hormones are noted in many studies.1
The short-term consequences of pain are well documented. An increase in HR, a decrease in SpO2, heart rate variability, blood pressure fluctuations and increased secretion of stress hormones are noted in many studies.1
Pain in the first few days of life has been shown to magnify the pain response to later stimuli.10
Pain in the first few days of life has been shown to magnify the pain response to later stimuli.10
Long-term Outcomes Associated with Neonatal Pain: A Timeline
Long-term Outcomes Associated with Neonatal Pain: A Timeline
40 WEEKS
More early “skin breaks” were associated with:
- reduced lateral thalamic volume
- slower metabolic thalamic growth
- altered/reduced white matter microstructure11,12

40 WEEKS
More early “skin breaks” were associated with:
- reduced lateral thalamic volume
- slower metabolic thalamic growth
- altered/reduced white matter microstructure11,12
8-18 MONTHS
Higher number of neonatal skin breaks predicted lower Mental Development Index13

8-18 MONTHS
Higher number of neonatal skin breaks predicted lower Mental Development Index13
3 YEARS
Cognitive and motor scores were predicted by thalamic volumetric growth, regardless of sex and GA at birth.11

3 YEARS
Cognitive and motor scores were predicted by thalamic volumetric growth, regardless of sex and GA at birth.11
7 YEARS
Cumulative neonatal pain-related stress was independently associated with changes in brain activity, which were negatively correlated with visual-perceptual abilities at school-age.9
Greater number of invasive procedures were associated with reduced white matter and lower IQ14
8 YEARS
More neonatal invasive procedures was associated with smaller amygdala and thalamus volumes, in turn related to poorer cognitive, visual-motor, and behavioral outcomes.15

7 YEARS
Cumulative neonatal pain-related stress was independently associated with changes in brain activity, which were negatively correlated with visual-perceptual abilities at school-age.9
Greater number of invasive procedures were associated with reduced white matter and lower IQ14
8 YEARS
More neonatal invasive procedures was associated with smaller amygdala and thalamus volumes, in turn related to poorer cognitive, visual-motor, and behavioral outcomes.15
Reducing painful procedures in the NICU is possible. Read the whitepaper.
- Kurdahi Badr et al. Newborn Infant Nurs Rev. 2013, 82-86.
- Kapellou et al. BMJ Clin Evid. 2009:0313.
- Simons et al. Arch Pediatr Adolesc Med. 2003;157(11):1058–1064.
- Roofthooft et al. Neonatology. 2014;105(3):218-226.
- Fitzgerald M. Nat Rev Neurosci 6. 2005. 507–520.
- Bellieni et al. J Matern. 2014. 29. 10.3109.
- Johnston et al. Cochrane Database of Syst Rev. 2017, Issue 2: CD008435.
- Shah et al. Cochrane Database Syst Rev. 2011;2011(10): CD001452.
- Doesburg et al. Pain. 2013;154(10):1946-1952.
- Gokulu et al. Acta Paediatr. 2016;105(11):e520-e525.
- Duerden et al. J Neurosci. 2018;38(4):878-886.
- Brummelte et al. Ann Neurol. 2012;71(3):385-396.
- Grunau et al. Pain. 2009;143(1-2):138-146.
- Vinall et al. Pediatrics. 2014;133(3):412-421.
- Chau et al. Front Behav Neurosci. 2019;13:51.
- Kurdahi Badr et al. Newborn and Infant Nursing Reviews, 2013, Pages 82-86,
- Kapellou et al. BMJ Clin Evid. 2009;2009:0313. Published 2009 Jan 7.
- Simons et al. Arch Pediatr Adolesc Med. 2003;157(11):1058–1064.
- Roofthooft et al. Neonatology. 2014;105(3):218-226.
- Fitzgerald M. Nat Rev Neurosci 6, 507–520 (2005).
- Bellieni et al. (2014). The Journal of Maternal-Fetal & Neonatal Medicine. 29. 10.3109
- Johnston et al. Cochrane Database of Syst Rev. 2017, Issue 2. Art. No.: CD008435.
- Shah et al. Cochrane Database Syst Rev. 2011;2011(10):CD001452. Published 2011 Oct 5.
- Doesburg et al. Pain. 2013;154(10):1946-1952.
- Gokulu et al. Acta Paediatr. 2016;105(11):e520-e525.
- Duerden et al. J Neurosci. 2018;38(4):878-886.
- Brummelte et al. Ann Neurol. 2012;71(3):385-396.
- Grunau et al.. Pain. 2009;143(1-2):138-146.
- Vinall et alPediatrics. 2014;133(3):412-421.
- Chau et al. Front Behav Neurosci. 2019;13:51.

